Pharmacovigilance
Clinical trials management system
Successful clinical trials require the ability to see key details and uncover hidden insights.
Medzinity utilizes science and technology to bring clarity to clinical trials, helping companies to develop new life-improving therapies more efficiently and safely.
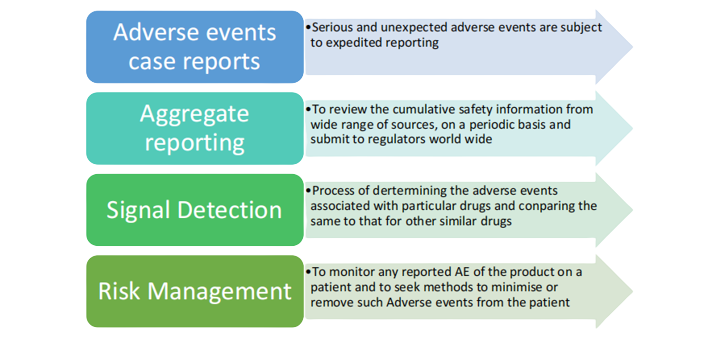
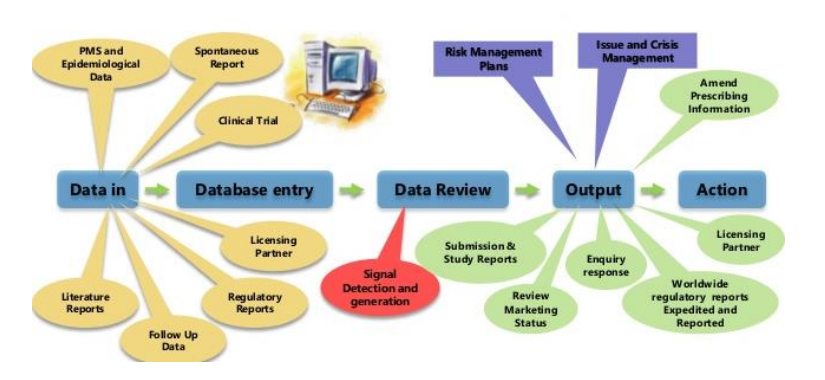
Pharmacovigilance operations/consulting
We support our clients throughout the medicine’s life cycle, starting from the establishment of the development program, continuing through the Marketing Authorization Application, and later during post marketing periodic reporting to regulatory authorities
Specialist support services
We provide specialist support services like risk management plan, responding to regular safety enquiries, assessment of benefit risk, safety concerns and safety communication, data safety monitoring boards and pharmacovigilance process development.
Regulatory affairs
We have capabilities to support end to end regulatory life cycle management across the entire value chain of drug and device regulatory environment.