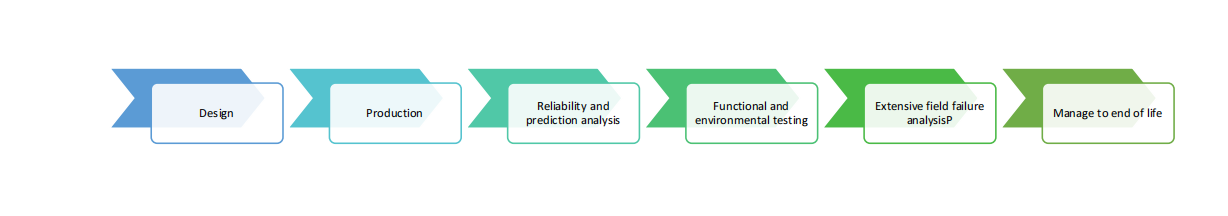
Product Life Cycle Management
Complaints handling
Complaint handling of products is done by our expert team using our analytical skills.
A brief summary of the complaint is triaged with all the relevant information pertaining to the complaint.
Cases are entered against the source documents received. Quality review and investigatory analysis follows the data entry in respective companies.
We provide a formalized, systematic solution to manage all aspects of product quality, reliability and risk using methods that are fully integrated into the product life cycle management.
Clinical evaluation Reports
Clinical evaluation is the assessment and analysis of clinical data pertaining to a medical device to verify its clinical safety and performance.
The evaluation is based on comprehensive analysis of pre- and post-market clinical data relevant to the intended use.
This includes data specific to the device as well as any data relating to devices claimed as equivalent by the manufacturer.
We have an expert team to document the whole process as well as conclusions of a clinical evaluation of devices.
Post marketing surveillance
We have extensive experience in post marketing surveillance management.
We prepare periodic safety update reports and do literature screening for safety assessments and benefit risk analysis.